C6H12O6 : Structure, Empirical Formula, and Polarity of Glucose
C6H12O6, commonly known as glucose, is a simple sugar that plays a crucial role in the energy production of living organisms. As the primary source of energy for cells, glucose is essential for various biological processes, including cellular respiration and metabolic pathways. Found naturally in fruits, vegetables, and honey, glucose is also a key component of carbohydrates, which the body breaks down to fuel its daily functions. Understanding the significance of C6H12O6 is fundamental to grasping how our bodies generate and utilize energy efficiently.
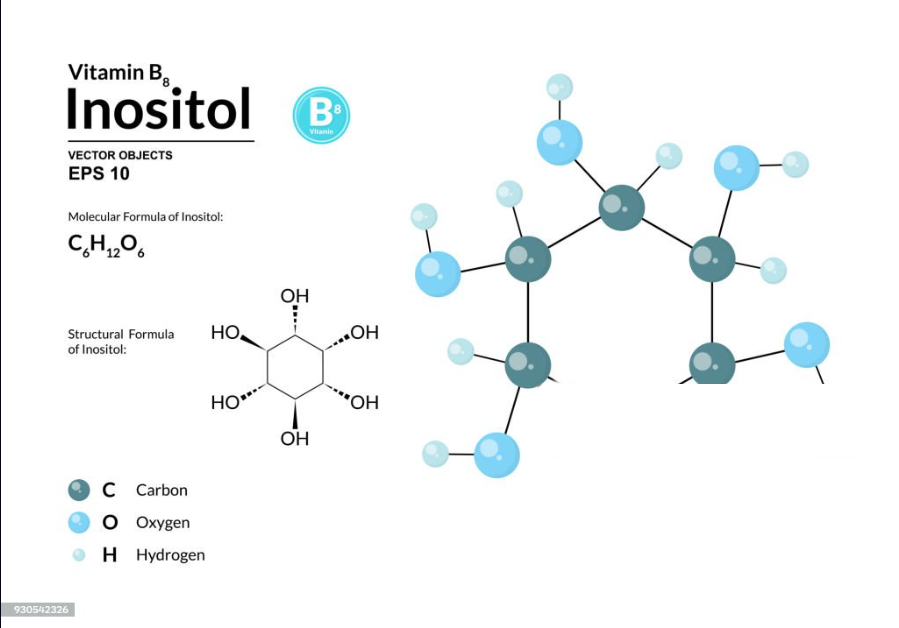
What is C6H12O6
Glucose is the chemical formula for glucose, a simple sugar and a vital carbohydrate in biology. Glucose is a monosaccharide, meaning it is one of the simplest forms of sugar, composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. It serves as a primary energy source for living cells and is crucial for various metabolic processes.
Glucose is produced by plants during photosynthesis and is a key energy source for both plants and animals. In humans, glucose is absorbed into the bloodstream from food and is used by cells for energy through a process called cellular respiration. The body maintains glucose levels in the blood within a narrow range, and imbalances can lead to conditions like hypoglycemia (low blood sugar) or hyperglycemia (high blood sugar), often associated with diabetes.
What is The Molar Mass of C6H12O6
The molar mass of C6H12O6 (glucose) can be calculated by summing the atomic masses of all the atoms in the molecule. Here’s how it’s done:
- Carbon (C): 666 atoms × 12.0112.0112.01 g/mol = 72.0672.0672.06 g/mol
- Hydrogen (H): 121212 atoms × 1.0081.0081.008 g/mol = 12.09612.09612.096 g/mol
- Oxygen (O): 666 atoms × 16.0016.0016.00 g/mol = 96.0096.0096.00 g/mol
Adding these together:
72.0672.0672.06 g/mol +++ 12.09612.09612.096 g/mol +++ 96.0096.0096.00 g/mol = 180.156180.156180.156 g/mol
So, the molar mass of glucose is approximately 180.16 g/mol.
Is C6H12O6 an Electrolyte
No, C6H12O6 (glucose) is not an electrolyte.
Electrolytes are substances that, when dissolved in water, dissociate into ions and conduct electricity. Glucose, being a neutral molecule with no charge, does not dissociate into ions when dissolved in water. Therefore, it does not conduct electricity and is not considered an electrolyte.
In contrast, substances like salts (e.g., sodium chloride, NaCl), acids (e.g., hydrochloric acid, HCl), and bases (e.g., potassium hydroxide, KOH) are electrolytes because they dissociate into ions in solution and can conduct an electric current.
Is C6H12O6 Ionic or Covalent
Glucose is a covalent compound.
In a covalent compound, atoms share electrons to form bonds, rather than transferring electrons as in ionic compounds. Glucose is made up of carbon, hydrogen, and oxygen atoms that are bonded together by covalent bonds. These atoms share electrons to achieve stable electron configurations, making glucose a covalent molecule.
What is The Molar Mass of Glucose C6H12O6
The molar mass of glucose (C6H12O6) is calculated by summing the atomic masses of all the atoms in the molecule:
- Carbon (C): 6 atoms × 12.01 g/mol = 72.06 g/mol
- Hydrogen (H): 12 atoms × 1.008 g/mol = 12.096 g/mol
- Oxygen (O): 6 atoms × 16.00 g/mol = 96.00 g/mol
Adding these together:
72.0672.0672.06 g/mol + 12.09612.09612.096 g/mol + 96.0096.0096.00 g/mol = 180.156180.156180.156 g/mol
So, the molar mass of glucose (C6H12O6) is approximately 180.16 g/mol.
Which Statement Correctly Describes Glucose c6h12o6
The correct statement that describes glucose (C6H12O6) is:
“Glucose is a simple sugar (monosaccharide) with the molecular formula C6H12O6, which serves as a primary source of energy for living organisms and is involved in various metabolic processes.”
This statement accurately captures glucose’s role, structure, and importance in biology.
A Molecule With the Chemical Formula C6h12O6 is Probably a
A molecule with the chemical formula C6H12O6 is probably a carbohydrate, specifically a monosaccharide or simple sugar.
Glucose is the most common example, but other sugars like fructose and galactose also share this chemical formula, making them isomers of each other. Monosaccharides are the simplest form of carbohydrates and serve as vital energy sources for living organisms.
How Many Elements Are in C6H12O6
The chemical formula C6H12O6 contains three different elements:
- Carbon (C)
- Hydrogen (H)
- Oxygen (O)
These three elements combine to form the glucose molecule, with the numbers indicating the quantity of each atom in the molecule: 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
Is C6H12O6 a Stronk Electrolyte ?
No, C6H12O6 (glucose) is not a strong electrolyte. In fact, it is not an electrolyte at all.
Electrolytes are substances that dissociate into ions when dissolved in water and can conduct electricity. Strong electrolytes completely dissociate into ions, such as sodium chloride (NaCl) or hydrochloric acid (HCl).
Glucose, on the other hand, is a covalent molecule that does not dissociate into ions in water. It dissolves as whole molecules, so it does not conduct electricity and is considered a non-electrolyte.
What Does the Formula C6H12O6 Mean
The formula C6H12O6 represents a molecule of glucose, which is a type of simple sugar or monosaccharide. Here’s what each part of the formula means:
- C6: Indicates the molecule contains 6 carbon atoms.
- H12: Indicates the molecule contains 12 hydrogen atoms.
- O6: Indicates the molecule contains 6 oxygen atoms.
In glucose, these atoms are arranged in a specific way to form a structure that is essential for various biological processes. Glucose is a primary energy source for cells in many organisms and plays a key role in metabolism.
What is the Empirical Formula for c6h12o6
The empirical formula of a compound represents the simplest whole-number ratio of the elements in that compound. To find the empirical formula for C6H12O6:
- Determine the greatest common divisor for the subscripts in the molecular formula (C6H12O6). Here, the greatest common divisor is 6.
- Divide each subscript in the molecular formula by this number:
- Carbon: 66=1\frac{6}{6} = 166=1
- Hydrogen: 126=2\frac{12}{6} = 2612=2
- Oxygen: 66=1\frac{6}{6} = 166=1
Thus, the empirical formula for C6H12O6 is CH2O.
What is the Empirical Formula of c6h12o6
The empirical formula of a compound represents the simplest whole-number ratio of the elements in that compound. For C6H12O6, the steps to determine the empirical formula are:
- Find the greatest common divisor of the subscripts (6, 12, and 6), which is 6.
- Divide each subscript by this greatest common divisor:
- Carbon: 66=1\frac{6}{6} = 166=1
- Hydrogen: 126=2\frac{12}{6} = 2612=2
- Oxygen: 66=1\frac{6}{6} = 166=1
Thus, the empirical formula of C6H12O6 is CH2O.
How Many Atoms Are In c6h12o6
To find the total number of atoms in a molecule of C6H12O6, you sum the number of each type of atom present in the formula:
- Carbon (C): 6 atoms
- Hydrogen (H): 12 atoms
- Oxygen (O): 6 atoms
Adding these together:
6 (C) + 12 (H) + 6 (O) = 24 atoms
So, a molecule of C6H12O6 contains a total of 24 atoms.
Is C6H12O6 a Compound
Yes, C6H12O6 is a compound.
A compound is a substance composed of two or more different elements chemically bonded together. In the case of C6H12O6, which is glucose, the molecule is made up of carbon, hydrogen, and oxygen atoms bonded together in a specific arrangement. This makes glucose a chemical compound.
Is C6H12O6 Organic or Inorganic
C6H12O6 (glucose) is an organic compound.
Organic compounds typically contain carbon atoms, often combined with hydrogen, oxygen, and other elements. Glucose, a simple sugar, includes carbon, hydrogen, and oxygen, making it a prime example of an organic compound. It plays a crucial role in biological processes and metabolic pathways. Organic compounds are generally linked to living organisms, and glucose is essential for energy production and metabolism in both plants and animals.
What is The Empirical Formula for The Compound C6H12O6
The empirical formula represents the simplest whole-number ratio of the elements in a compound. For the molecular formula C6H12O6, follow these steps to find the empirical formula:
- Find the greatest common divisor for the subscripts of the elements. Here, the greatest common divisor for 6, 12, and 6 is 6.
- Divide each subscript in the molecular formula by this greatest common divisor:
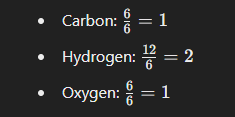
Thus, the empirical formula for C6H12O6 is CH2O.
What is The Empirical Formula Of Glucose C6H12O6
The empirical formula of glucose (C6H12O6) is CH2O.
To determine this:
- Identify the number of each type of atom in the molecular formula:
- Carbon: 6 atoms
- Hydrogen: 12 atoms
- Oxygen: 6 atoms
- Find the greatest common divisor of the subscripts (6, 12, and 6), which is 6.
- Divide each subscript by this greatest common divisor:
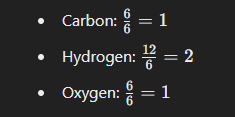
Thus, the empirical formula is CH2O.
How Many Atoms Are in C6H12O6?
To determine the total number of atoms in a C6H12O6 molecule, you need to count each type of atom:
- Carbon (C): 6 atoms
- Hydrogen (H): 12 atoms
- Oxygen (O): 6 atoms
Add these numbers together:
6 (C) + 12 (H) + 6 (O) = 24 atoms
So, a glucose molecule contains a total of 24 atoms.
How Many Different Elements Are in the Compound C6H12O6?
Glucose contains three different elements:
- Carbon (C)
- Hydrogen (H)
- Oxygen (O)
These elements combine to form glucose.
What Does C6H12O6 Stand For?
The formula C6H12O6 represents glucose, a simple sugar and a crucial monosaccharide in biology. Scientists use glucose as a primary source of energy for cells in many organisms. Glucose also plays a key role in various metabolic processes.
In the formula:
- C6 indicates six carbon atoms.
- H12 indicates twelve hydrogen atoms.
- O6 indicates six oxygen atoms.
“Cells break down glucose during cellular respiration to produce energy.”
Is C6H12O6 an Organic Molecule?
Yes, glucose is an organic molecule. Organic molecules feature carbon atoms, often bonded with hydrogen, oxygen, and other elements. As a type of simple sugar or monosaccharide, glucose contains carbon, hydrogen, and oxygen. It plays a fundamental role in biological processes, including cellular respiration and metabolism, and is a key component in many biological systems.
What is C6H12O6 Called?
The chemical formula glucose represents glucose, a type of simple sugar or monosaccharide. Glucose serves as an important carbohydrate and a primary energy source for cells in many organisms. It plays a critical role in various metabolic processes.
Is C6H12O6 an Empirical Formula?
No, C6H12O6 is not an empirical formula; it is the molecular formula for glucose.
The empirical formula shows the simplest whole-number ratio of the elements in a compound. For glucose, the empirical formula is CH2O. This formula reveals the simplest ratio of carbon, hydrogen, and oxygen atoms in glucose.
Is C6H12O6 Polar or Nonpolar?
C6H12O6 (glucose) is a polar molecule.
Here’s why:
- Polar Covalent Bonds: The bonds between carbon and oxygen in glucose are polar covalent. Since oxygen is more electronegative than carbon, it creates partial negative charges on the oxygen atoms, while the carbon atoms develop partial positive charges.
- Molecular Structure: Glucose contains multiple polar hydroxyl groups (-OH). The glucose molecule contains multiple polar hydroxyl groups (-OH), and these groups increase its overall polarity.
- Asymmetrical Distribution: The arrangement of carbon, hydrogen, and oxygen atoms in glucose leads to an asymmetrical charge distribution. This results in a net dipole moment, making glucose a polar molecule.
Due to its polar nature, glucose interacts well with water and other polar substances
Click to browse our other articles and categories.
A resource that provides more information about the medical uses of glucose. Resource : https://diabetes.org
An article or educational resource that provides information about photosynthesis and glucose production. Source : https://www.khanacademy.org/science/ap-biology/cellular-energetics/photosynthesis
